FectoVIR®-AAV: Revolutionizing Recombinant AAV-In the vast landscape of biotechnology, where innovation is the key to progress, FectoVIR®-AAV emerges as a groundbreaking synthetic transfection reagent, orchestrating a symphony of advancements in the industrial-scale production of recombinant Adeno-Associated Virus (rAAV).
Join us as we delve into the intricate details of this technological marvel and explore how it transforms the AAV manufacturing process.
FectoVIR®-AAV: A Technological Marvel:
At the core of FectoVIR®-AAV lies a commitment to process economics, industrial scalability, and GMP-grade availability, making it a game-changer in the world of biopharmaceuticals.
Let’s unravel its capabilities and understand how it’s reshaping the landscape of rAAV production.
Process Economics: Elevating Productivity, Reducing Costs:
FectoVIR®-AAV takes center stage by significantly expanding the number of doses per batch, a feat achieved through its high AAV productivity.
By setting a new standard for manufacturing cost reduction, this synthetic transfection reagent outshines competitors, providing up to a remarkable 10-fold increase in viral genome titer compared to existing alternatives.
The implications are profound, as higher titers directly translate to a more cost-effective production process.
Industrial Scalability: Breaking Barriers, Redefining Possibilities:
Designed for large-scale production, FectoVIR®-AAV redefines industrial scalability.
Overcoming volume constraints that often hinder large-scale transient transfection, this innovative reagent optimizes complexation volume down to 1%. This breakthrough not only streamlines the preparation of transfection complexes but also reduces the footprint, paving the way for lower capital expenses and more efficient manufacturing processes.
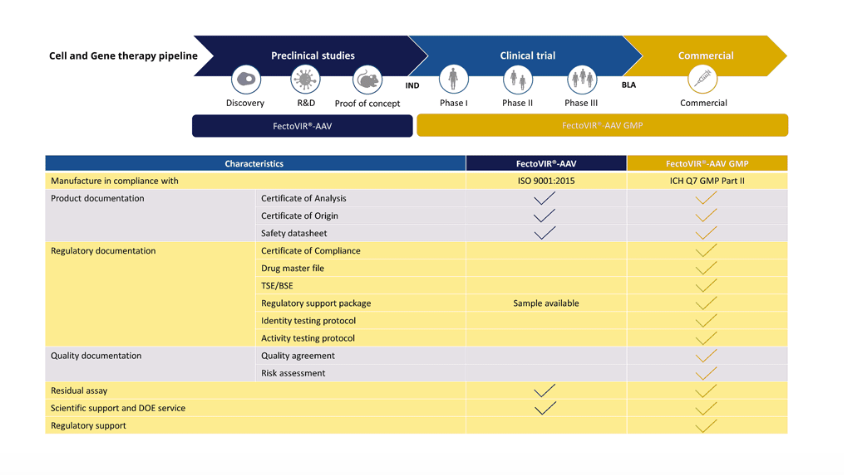
Ensuring Patient Safety: The GMP Standard:FectoVIR®-AAV:
In the realm of biopharmaceuticals, patient safety is paramount. FectoVIR®-AAV doesn’t just excel in performance; it is GMP-grade, manufactured in compliance with ICH Q7 guidelines.
This ensures a validated and aseptic manufacturing process, aligning with the highest quality standards for active pharmaceutical ingredients (API).
The product is available in a 1 L bag, enabling a closed system with MPC connection and weldable c-flex tubing, thus minimizing contamination risks.
Also Read:jetPRIME, jetOPTIMUS, jetPEI , in vivo-jetRNA , FectoVIR-LV.